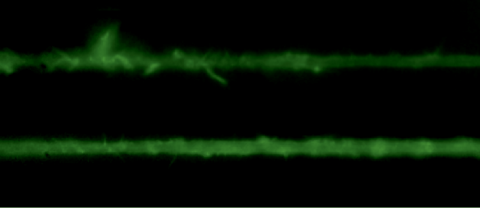
Mecánica Celular
The Cell Mechanics Laboratory of the Physics Department develops research on the physical properties that emerge from biological activity. Our interest is focused on the dynamics of cell adaptation in the face of external forces. Another topic of interest is the detection of biological activity in systems where thermal effects predominate, such as the red blood cell membrane. To address these problems of physical and biological interest, we build and develop ad hoc experimental techniques such as optical tweezers, fluctuation detection by laser interferometry that allows us to obtain large volumes of data to characterize these systems.
|
|
Thermal fluctuations of biological systemsBiological systems are characterized by large out-of-equilibrium dynamics where broken detailed balance at the molecular scale induce… coming soon |
|
|
Actin cytoskeleton dynamicsOne of the many effects of soft tissues under mechanical solicitation in the cellular damage produced by high localized strain. We are interested to study the response of peripheral Stress Fibers (SFs) to external stretch in mammalian cells, plated onto deformable micro-patterned substrates. A local fluorescence analysis reveals that an adaptation response is observed at the vicinity of the Focal Adhesion sites (FAs) due to its mechano-sensor function. The response depends on the type of mechanical stress, from a Maxwell type material in compression to a complex scenario in extension, where a mechano-transduction and a self-healing process takes place in order to prevent the induced severing of the SF. A model is proposed to take into account the effect of the applied stretch on the mechanics of the SF from which relevant parameters of the healing process are obtained. In contrast, the repair of the actin bundle occurs at the weak point of the SF and depends on the amount of the applied strain. As a result, the SFs display strain-softening features due to the incorporation of new actin material into the bundle. In contrast, the response under compression shows a reorganization with a constant actin material suggesting a gliding process of the SFs by the Myosin II motors. https://www.mdpi.com/1422-0067/23/9/5095
|
|
|
Neurite mechanics and biological functionThermal Fluctuations Spectroscopy (TFS) in combination with novel optical-based instrumentation was used to study mechanical properties of cell-cultured neurites with a spatial resolution limited only by the light diffraction. The analysis of thermal fluctuations together with a physical model of cellular elasticity allows us to determine relevant mechanical properties of neurite as axial tension, flexural rigidity, plasma membrane tension, membrane bending rigidity and cytoskeleton to membrane-coupling, whose values are consistent with previously reported values measured using invasive approaches. These measurements have the advantage of not requiring the application of an external force, allowing as to directly establish a correlation between changes in the mechanical parameters and cytoskeleton-protein concentrations. Axonal beading, or the formation of a series of swellings along the axon, and retraction are commonly observed shape transformations that precede axonal atrophy in Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative conditions. The mechanisms driving these morphological transformations are poorly understood. By making quantitative analysis of the shape modes under different conditions, measurement of membrane tension, and using theoretical considerations, we argue that membrane tension is the main driving force that pushes cytosol out of the axon when microtubules are degraded, causing axonal thinning. Under pharmacological perturbation, atrophy is always retrograde, and this is set by a gradient in the microtubule stability. The nature of microtubule depolymerization dictates the type of shape transformation, vis-à-vis beading or retraction. Elucidating the mechanisms of these shape transformations may facilitate development of strategies to prevent or arrest axonal atrophy due to neurodegenerative conditions. https://www.frontiersin.org/articles/10.3389/fncel.2018.00194/ |